- The spider in the web -
An introduction to HFE
“UI design plays and important role in causing use error and/or preventing it. Use errors are to be expected and should be identified as part of overall device risk which necessitates specific ”human factors” considerations.” –Ron Kaye, FDA
Why Medical Device (MD) manufacturers should consider applying good Human Factors Engineering (HFE) principles into their Design & Development process is an often heard question. Nobody wants yet more rules and regulations to adhere to so, the added value of HFE is often underrated and the regulatory necessity underestimated. Go to the HFE principles page to quickly learn how HF engineering can be integrated into your development processes.
The very short answer to the question why you should consider HFE is that: designing for safe usability matters for Patient Safety. The slightly longer answer why HFE should be applied is:
- It is the law! European and USA (FDA) regulations relevant to Medical Devices (MDD, MDR, IVDR, IEC-62366, MHRA and FDA) ALL stipulate the need to show evidence of use centered design for all devices where use errors can result in patient harm.
- It will help you with successful, first-time-right pre-market submissions for both the European and USA and beyond.
- It will reduce the chance of product recalls since you discovered the unanticipated use errors during design in stead of after launch.
- For product redesign, it is a powerful tool to help turn known use problems into safer design solutions.
A large number of medical devices are used for critical patient monitoring, and errors in use, leading to patient harm, have progressively become a main cause for concern for regulators, manufacturers and patients. The cause of such errors can often be due to poorly designed device user interfaces, particularly where a complex user system is involved. Medical devices, used in busy environments with many distractions, have become more and more diverse in their capabilities and complexity. As patient care evolves and is transferred to private homes or public environments, often less skilled or even unskilled users including patients and caretakers must be enabled to safely use these complex devices.
Spider in the web
At Weaver we view the Human Factors Engineer as a valuable spider in your R&D web. The Human Factors Engineer is one of the few team members that frequently spends time with the intended users and understands their needs, work environment, (bad) habits and constraints. Feeding that information back into you R&D organization allows you to continuously verify that your design is moving in the right direction and your product will be safe, effective and successful in the environment where you want to deploy it.
Connecting people
Uniquely positioned in between Design, Development and the real world, the HF engineer can create bridges between users and designers and within your development organization to help your staff understand and appreciate the true way the product will be used. Understanding the challenges real users face will help them to make the right design choices and build a better, and safer product.
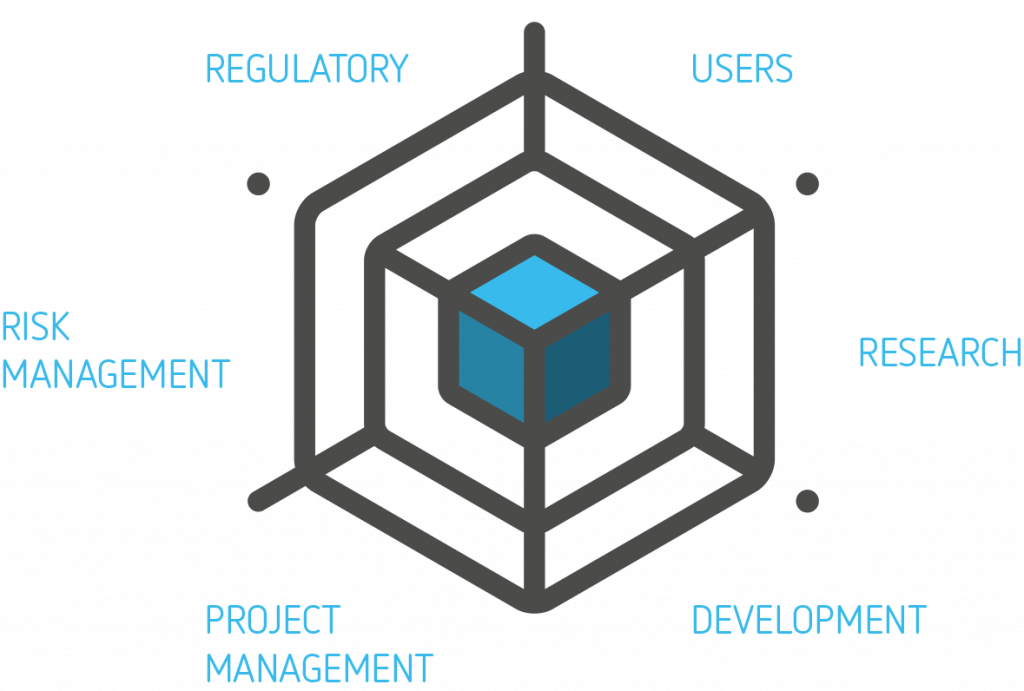
With the product and the user in the center of the web, the HF engineer observes USERS and works with RISK MANAGEMENT to identify risks and risk mitigations which are translated, together with your RESEARCH team, into use related safety requirements. During DEVELOPMENT the HF engineer can evaluate design (prototypes), allowing the teams to quickly identify usable design solutions and progress with confidence. The HFE process is designed to detect the big OOPSES early, helping PROJECT MANAGEMENT to reduce the chance of costly, late design changes. The end result is a safe and effective medical device that allows REGULATORY to obtain pre-market approval, and you to have a successful market introduction.
